Types of Battery Chemistries
Battery chemistries are the heart of modern energy storage solutions, powering our devices, vehicles, and even homes. These chemistries represent diverse technologies, each with unique materials and mechanisms.
Lithium-ion batteries dominate portable electronics and electric vehicles due to their high energy density and longevity. Lead-acid batteries remain pivotal in automotive and backup power applications with their reliability.
Nickel-cadmium and nickel-metal hydride batteries offer alternatives with good cycle life and lower environmental impact.
Alkaline batteries, with their zinc and manganese dioxide composition, are cost-effective and widely used.
Emerging technologies like solid-state and sodium-ion batteries hold promise for future advancements in energy storage.
Understanding these chemistries is essential for harnessing the power that drives our modern world.
There are several types of battery chemistries, each with its own working principles, anode and cathode materials, chemical formulas, and advantages. Here are some of the most common battery chemistries:
1. Lithium-ion (Li-ion) Batteries

Working: Li-ion batteries use lithium ions to move between the anode (typically made of graphite) and the cathode (usually made of lithium cobalt oxide, lithium iron phosphate, or other materials). During discharge, lithium ions move from the anode to the cathode, creating an electrical current.
Anode Material: Graphite
Cathode Material: Various materials like lithium cobalt oxide (LiCoO2), lithium iron phosphate (LiFePO4), lithium manganese oxide (LiMn2O4), etc.
Chemical Formula: Various, depending on the cathode material.
Advantages:
- High energy density
- low self-discharge
- long cycle life
- lightweight
- Widely used in portable electronics and electric vehicles.
2. Lead-Acid Batteries:

Working: Lead-acid batteries utilize lead dioxide as the cathode and sponge lead as the anode immersed in a sulfuric acid electrolyte. During discharge, lead and lead dioxide react with sulfuric acid to produce electricity.
Anode Material: Sponge lead (Pb)
Cathode Material: Lead dioxide (PbO2)
Chemical Formula: Pb + PbO2 + 2H2SO4 → 2PbSO4 + 2H2O
Advantages:
- Low cost,
- high discharge currents
- well-established technology
- Widely used in automotive and backup power applications.
3. Nickel-Cadmium (NiCd) Batteries

Working: NiCd batteries have a nickel hydroxide cathode and a cadmium anode separated by a potassium hydroxide electrolyte. During discharge, nickel hydroxide and cadmium react to produce electricity.
Anode Material: Cadmium (Cd)
Cathode Material: Nickel hydroxide (Ni(OH)2)
Chemical Formula: Cd + 2Ni(OH)2 → Cd(OH)2 + 2NiOOH
Advantages:
- Good cycle life,
- high discharge currents
- Relatively low cost, but they suffer from the “memory effect.”
4. Nickel-Metal Hydride (NiMH) Batteries
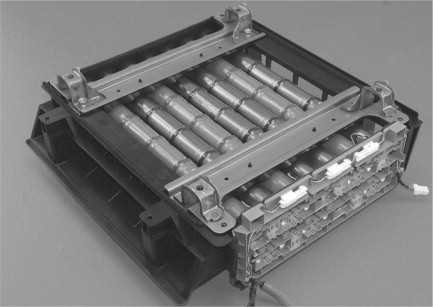
Working: NiMH batteries have a nickel oxyhydroxide cathode and a hydrogen-absorbing alloy anode, separated by a potassium hydroxide electrolyte. During discharge, hydrogen ions are released from the alloy and combine with nickel oxyhydroxide to produce electricity.
Anode Material: Hydrogen-absorbing alloy
Cathode Material: Nickel oxyhydroxide (NiOOH)
Chemical Formula: Various, depending on alloy and cathode material.
Advantages:
- Higher energy density than NiCd,
- No cadmium (environmentally friendlier), and less susceptible to memory effect.
5. Alkaline Batteries
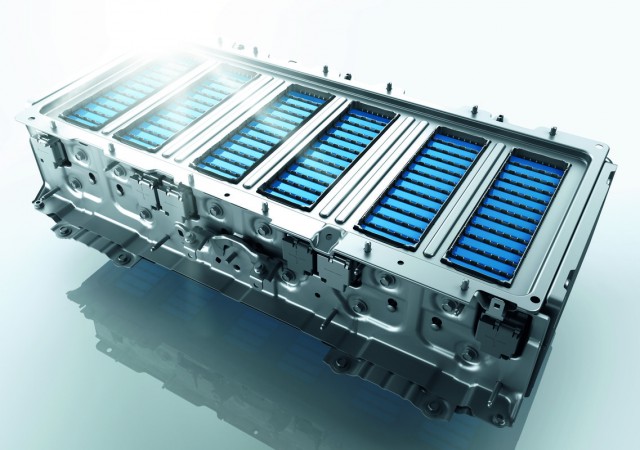
Working: Alkaline batteries have a zinc anode and a manganese dioxide cathode, with potassium hydroxide (alkaline) electrolyte. During discharge, zinc reacts with manganese dioxide to produce electricity.
Anode Material: Zinc (Zn)
Cathode Material: Manganese dioxide (MnO2)
Chemical Formula: Zn + 2MnO2 → ZnO + 2Mn2O3
Advantages:
- Inexpensive
- widely available
- longer shelf life than zinc-carbon batteries
- Suitable for a wide range of applications.
Comparison table of various battery chemistries, including Lithium-ion, Lead-Acid, Nickel-Cadmium (NiCd), Nickel-Metal Hydride (NiMH), and Alkaline batteries, based on different parameters:
Comparison Types of Battery Chemistries
| Parameter | Lithium-Ion | Lead-Acid | Nickel-Cadmium (NiCd) | Nickel-Metal Hydride (NiMH) | Alkaline |
|---|---|---|---|---|---|
| Energy Density (Wh/kg) | High | Moderate | Moderate | Moderate | Moderate |
| Cycle Life | High | Moderate | Moderate | Moderate | Moderate |
| Self-Discharge Rate | Low | Moderate | High | Moderate | Low |
| Environmental Impact | Low | Moderate to High | Moderate to High | Moderate to High | Moderate to High |
| Cost | Moderate to High | Low to Moderate | Moderate | Moderate | Low |
| Common Applications | Electronics, EVs | Automotive, Backup, Power, UPS | Portable Electronics, Power Tools, Aviation | Portable Electronics, Power Tools, Medical Devices | General Purpose, Devices, Toys |
| Anode Material | Graphite | Lead | Cadmium | Hydrogen-absorbing alloy | Zinc |
| Cathode Material | Various | Lead Dioxide | Nickel Hydroxide | Nickel Oxyhydroxide | Manganese Dioxide |
| Typical Voltage (V) | 3.6 – 3.7 | 2.0 – 2.2 | 1.2 | 1.2 | 1.5 |
Read More:- Lithium Nickel Manganese Battery: Working Process and Advantages










