
Lithium Iron Phosphate Battery
Lithium Iron Phosphate (LiFePO4 or LFP) batteries are a type of rechargeable lithium-ion battery known for their high energy density, long cycle life, and enhanced safety characteristics. Lithium Iron Phosphate (LiFePO4) batteries are a promising technology with a robust chemical structure, resulting in high safety standards and long cycle life. Their cathodes and anodes work in harmony to facilitate the movement of lithium ions and electrons, allowing for efficient charge and discharge cycles. These batteries have found applications in electric vehicles, renewable energy storage, portable electronics, and more, thanks to their unique combination of performance and safety.
Chemical Formula:
The chemical formula for a Lithium Iron Phosphate battery is:
LiFePO4.
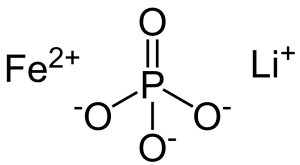
This formula is representative of the core chemistry of these batteries, with lithium (Li) serving as the primary cation, iron (Fe) as the transition metal, and phosphate (PO4) as the anion.
The specific arrangement and chemical reactions within the battery involve multiple phases and materials, but the fundamental chemistry revolves around these components.
Working of Lithium Iron Phosphate Battery:
Electrochemical Reactions
LiFePO4 batteries operate on the principles of electrochemistry, involving the movement of lithium irons between the cathode and anode during charge and discharge cycles.
Charging (Discharge):
- At the anode (negative electrode), during charging, lithium irons are extracted from the cathode material (LiFePO4) and intercalated into the anode material, typically graphite. This reaction can be represented as: LiFePO4 ⟶ LiFePO4-x + xLi^+ + xe^-
- The electrons released during this process flow through an external circuit, producing electrical energy that can be used to power various devices.
- At the cathode (positive electrode), the lithium irons migrate through the electrolyte and separator, reaching the anode. Here, they are intercalated into the anode material (graphite), stored as Li_xC6, releasing energy in the form of electrical potential.
Discharging (Charge):
- During discharging, the stored energy is released as lithium irons migrate from the anode to the cathode, allowing for the flow of electrons through the external circuit, thereby powering devices.
- At the cathode, LiFePO4 accepts lithium irons and electrons to form LiFePO4-x. This process stores energy in the cathode material.
- The overall reaction is reversible, enabling multiple charge and discharge cycles.
Selection of Cathode and Anode for Lithium Iron Phosphate Batteries:
Cathode (Positive Electrode):
- The cathode in a LiFePO4 battery is typically made of lithium iron phosphate (LiFePO4). This material has several advantages, including:
- High thermal and chemical stability, contributing to the battery’s safety.
- Low cost and environmental friendliness due to the absence of toxic or rare materials.
- Good cycle life, allowing for a high number of charge and discharge cycles.
- Stable voltage profile, making it suitable for applications requiring constant voltage.
Anode (Negative Electrode):
- The anode in LiFePO4 batteries is commonly made of graphite. Graphite provides a stable and reversible platform for the intercalation of lithium irons during charging and discharging.
- High electrical conductivity, facilitating efficient electron transfer.
- Excellent lithium-ion intercalation properties.
- Stability over numerous charge and discharge cycles.
Some Advantages of Lithium Iron Battery:

- Safety: LiFePO4 batteries have a lower risk of thermal runaway and are less prone to overheating, making them safer for various applications, including electric vehicles.
- Long Cycle Life: LiFePO4 batteries can endure a significantly higher number of charge and discharge cycles, often exceeding 2000 cycles, before showing significant capacity degradation.
- High Energy Density: While not as high as some other lithium-ion chemistries, LiFePO4 batteries offer a good balance between energy density and safety.
- Environmental Friendliness: LiFePO4 batteries contain non-toxic materials, making them more environmentally friendly and easier to recycle.
Read More:- Types of Batteries Used in Electric Vehicles in India









