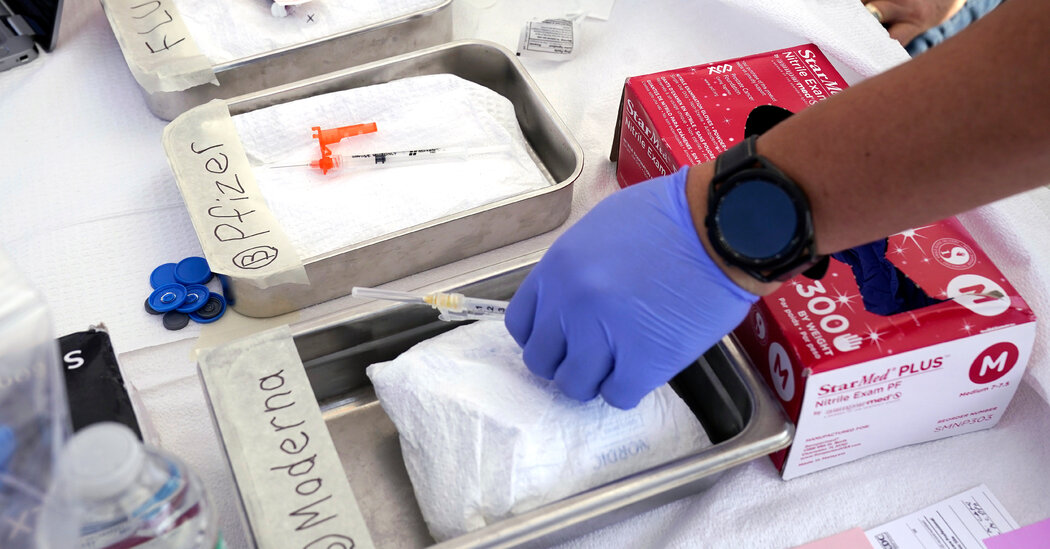
The latest Covid boosters are expected to be approved by the Food and Drug Administration as early as Monday, arriving alongside the seasonal flu vaccine and shots to protect infants and older adults from R.S.V., a potentially lethal respiratory virus.
The Centers for Disease Control and Prevention is expected to follow up on Tuesday with an advisory meeting to discuss who should get the new shots, by Pfizer-BioNTech and Moderna. After a final decision by the C.D.C.’s director, millions of doses will be shipped to pharmacies, clinics and health systems nationwide within days.
As Covid cases creep up, the prevention measures could portend the first winter of the decade without a crush of patients pushing hospitals beyond capacity. But a healthy winter is far from a lock: Last year, the updated Covid vaccine made it into the arms of only 20 percent of adults in the United States.
Some experts view that statistic with little alarm because the number of Covid deaths slowed over the last year, thanks to an increasingly immune population and higher vaccine rates among older Americans. Others see this year as an opportunity to protect more vulnerable people from severe illness or death.
“We now have some really good tools,” said Dr. Marcus Plescia, chief medical officer of the Association of State and Territorial Health Officials, a public health group. “It’s just — what is it going to take to get people comfortable with using them?”
Federal officials have been retreating from labeling the new formulation as boosters to previous shots, preferring to recast them as an annual immunization effort akin to the flu vaccine. That shift may reflect concern over the fatigue that some Americans have expressed about yet another round of shots against the virus.
The vaccine campaign will also be the first since the end of the public health emergency, which expired in May. In previous years, the U.S. government bought hundreds of millions of vaccine doses and distributed them for free. This year, private insurance and government payers like Medicare that cover the vast majority of Americans are expected to provide the vaccines to people for no fee.
But the question remains whether the private market of hospitals, clinics and pharmacies will be able to calibrate their vaccine orders to stock a realistic supply. Experts are uncertain how much demand there will be for the latest shots.
“There could be a period in here where things are a little bit chaotic, and that’s never a good situation,” Dr. Plescia said.
Also of concern in the handoff to the private market: the nation’s 23 million adults with no health insurance. The Biden administration has made plans to cover costs and offer the Covid vaccine through local clinics and major pharmacies, but some experts are worried about whether people who lack insurance will be aware of the new shots — or where to get them.
“They don’t have an insurer sending them leaflets — they may not have a usual source of care,” said Anthony Wright, executive director of Health Access, a California advocacy group. “And so the trusted messenger of their health plan, their doctor, their clinic, is not there saying, ‘It’s no cost. It’s really easy.’”
Vaccine manufacturers are expected to donate doses for the uninsured. Kelly Cunningham, a spokeswoman for Moderna, said the company had no cap on the number of Covid vaccine doses it planned to donate.
The latest shots are becoming available as Covid hospitalizations and deaths are rising slightly, albeit not to the levels of past years. In the week ending Aug. 26, there were 17,400 people admitted to the hospital — more than about 6,000 at a low point this summer. Deaths were also up to about 600 a week last month, though far lower than the weekly average of 14,000 deaths of 2021.
Once the vaccines are approved and the C.D.C. signs off, the Biden administration plans to urge the public to get their Covid and flu shots at the same time, a practice that has been studied and deemed safe, an administration official said. It’s a messaging effort they expect to share with major vaccine makers, which will be marketing the Covid doses commercially for the first time.
Walgreens and CVS said they both already have the updated flu and R.S.V. shots available in stores. Once Covid vaccine approvals are in place, Dr. Kevin Ban, Walgreens’ chief medical officer, said the chain would have the new shots on hand “as soon as possible.” A CVS spokesperson said doses could be arriving later this week. Representatives of both chains said the Covid shot would be available at no cost to all who are eligible under the C.D.C. guidelines expected Tuesday.
Targeted populations most certainly will include people 65 and older as well as those who are immunocompromised or have serious underlying medical conditions that leave them more susceptible to severe illness from the virus.
Nursing homes, some of which were host to inoculation teams from the major drugstore chains when vaccines first became available, are now relying on their usual long-term-care pharmacies to supply most vaccines. But many homes have fallen behind on booster rates: Recent Medicare data show that about 62 percent of residents are up-to-date on their shots even though older adults are among the most vulnerable to severe disease and death from the virus.
The new Covid vaccines target the XBB.1.5 variant, which was dominant when vaccine makers began to formulate and test a new version. Though the virus has had a rotating cast of variants, experts say the new Covid jab should fortify protections against severe infection.
Recent fears that one newer, highly mutated variant would escape the vaccine proved unfounded by reputable independent labs, said Fikadu Tafesse, an associate professor of molecular microbiology and immunology at Oregon Health & Science University. The C.D.C. also reviewed studies on the matter and confirmed Friday that the vaccine was holding strong.
“We were really getting ready for no response at all, but the data is very, very promising,” Dr. Tafesse said.
As with earlier shots, the updated ones are not expected to eliminate the chances of contracting a mild case of Covid. Instead, they are expected to reduce the chances of severe illness, hospitalization or death. The first Covid vaccines, given in early 2021 and targeting the initial form of the virus that emerged in Wuhan, had an efficacy rate of about 95 percent, meaning that far fewer vaccinated people became sick than those who were not immunized.
As the first vaccine’s potency waned with newer Omicron variants, a bivalent booster was approved in August 2022 that targeted the initial virus and BA.5, which was dominant at the time. That shot led to fewer people with Covid being hospitalized, dropping over several months to 25 percent from 60 percent..
The latest mRNA vaccines by Pfizer and Moderna is called a monovalent because it was aimed at one variant of Omicron, XBB.1.5., and unlike earlier boosters does not include protection against the original virus that caused widespread infections in China more than three years ago. But experts and researchers say that it should provide protection against many of Omicron’s variants.
Pfizer and Moderna reported that their vaccines had a potent response to the newest circulating variants, though only Moderna posted its initial data on Thursday.
But researchers continue to discuss how well it will stand up to new variants. The F.D.A. has mainly reviewed results submitted by the companies of animal or smaller human studies of immune response.
Jerica Pitts, a spokeswoman for Pfizer, said the data submitted by the company to the F.D.A. in June involved tests in animals. Trials following people who received the shot are continuing, she said.
Moderna submitted data to the F.D.A. on the immune response of 100 people to the new shots, which the company said in June “robustly elicit neutralizing antibodies” against XBB variants.
John Moore, a professor of virology and immunology at Weill Cornell Medicine, said he was not impressed with the latest results. He said the new shot showed an immune response similar to last fall’s booster. That means that although the new shot will be worth getting, “it’s nothing remotely like a game changer.”
Regulators are also considering whether to authorize a booster dose from Novavax, which employs a different but widely used technology for its coronavirus vaccine. That shot could be authorized in the coming weeks, giving some Americans who may prefer Novavax’s formulation as an alternative to the vaccines offered by Moderna and Pfizer-BioNTech.
Dr. Daniel Griffin, an infectious disease physician at Columbia University in New York, said getting the Covid shot in late October would provide robust protection at a time when people gather for holidays, and would help stop the virus’s spread to the most vulnerable, including older adults, pregnant people and those with compromised immune systems.
And while many might be weary of the social-protection argument, he said they could lessen their own odds of a more serious outcome.
“So a younger individual may say, ‘I’m not going to get a booster for the public health,’” Dr. Griffin said, “‘but I am going to get a booster because if I can reduce my chance of getting Covid, I can reduce my chance of long Covid.’”
Noah Weiland and Carl Zimmer contributed to this report.