The prestigious Lasker Awards were given on Thursday to scientists making advances in the diagnosis of eye disease, the prediction of cellular protein structure and the intricacies of the immune system. The awards, closely watched by researchers in biomedical fields, often foreshadow Nobel Prizes.
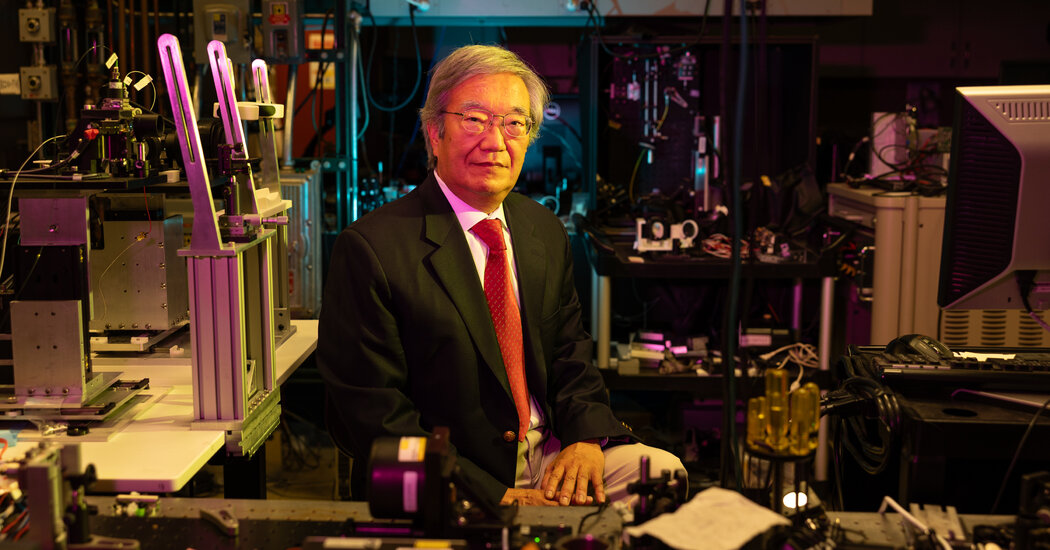
The Lasker-DeBakey Clinical Medical Research Award was given to a team of three scientists, led by James G. Fujimoto, a professor of electrical engineering at the Massachusetts Institute of Technology, who helped invent optical coherence tomography.
The technology can detect conditions like macular degeneration and diabetic retinopathy earlier than previous methods, preventing blindness. O.C.T. now is commonly used in ophthalmology offices, where the patient simply rests a chin and forehead against an instrument for a brief scan.
The method, invented in 1991, offers a staggering amount of detail about the retina, a layer of tissue at the back of the eye critical to vision.
“It’s not much thicker than a strand of hair, but it has 10 internal layers,” said Dr. David Huang, an ophthalmologist at the Casey Eye Institute at Oregon Health & Science University who helped to invent the method.
Before O.C.T., an ophthalmologist could dilate a patient’s eyes with eye drops to enlarge the pupil, and then use a magnifying lens and special light to examine the retina. An O.C.T. scan “can measure the thickness of the retina, the fluid pockets in it and the abnormal blood vessel growth,” detecting small lesions that have not yet caused symptoms, Dr. Huang added.
He estimated that roughly 40 million O.C.T. exams were done worldwide each year.
Eric A. Swanson, an M.I.T. researcher and a member of the team that invented the technique, noted that the instruments used today for O.C.T. have built on enormous advances.
“Systems are thousands of times faster today,” Mr. Swanson said. “They’re higher resolution, they’re more automated, they’re more high definition.”
O.C.T. tests are typically much cheaper than CT scans or other technologies for examining the body in fine detail. O.C.T. has been used to inspect arteries, an indication of how it can be applied elsewhere in the body.
Cellular Origami
In 2020, the London-based artificial intelligence lab DeepMind solved what was called “the protein folding problem.” The work, led by Demis Hassabis, the company’s chief executive, and John Jumper, a researcher, received the Albert Lasker Basic Medical Research Award.
Proteins are workhorses in the cellular machinery of all living things, including viruses, bacteria and the human body. They begin as strings of chemical compounds before folding into three-dimensional shapes that define what they can and cannot do.
Pinpointing a protein’s shape is often important to fighting disease and developing new therapies. A bacterium might resist an antibiotic by creating a particular protein, for example. If scientists can determine its shape, they might bypass this resistance.
For decades, this task required extensive experimentation involving X-rays, microscopes and other physical tools in the lab. Then Dr. Jumper and other DeepMind researchers built AlphaFold, an A.I. system that can be given the string of amino acids that make up a given protein and reliably predict its shape in a matter of minutes.
A year later, DeepMind freely shared the tool with scientists across the globe, instantly supercharging efforts to understand diseases, develop new medicines and explore various mysteries of the human body. By 2022, the lab had released predictions for nearly every protein known to science — more than 200 million proteins, observed in a million species.
Some scientists have used the technology to better understand the coronavirus. Others have used AlphaFold to predict how viruses will mutate or how proteins are discarded by the body — both of which can affect the progress of diseases and other ailments.
In recent months, researchers have turned to this kind of technology to generate entirely new proteins that do not exist in nature, as a way of accelerating drug discovery. “I am amazed by how many different and creative ways that people are using it,” Dr. Jumper said in an interview.
Dodging the Body’s Defenses
The Lasker-Koshland Special Achievement Award in Medical Science went to Dr. Piet Borst, a biochemist at the Netherlands Cancer Institute known for his discoveries about the wiliness of certain parasitic diseases and the resistance of cancer cells to chemotherapy medications.
The author of more than 400 articles, Dr. Borst discovered that parasites, including trypanosomes, the cause of African sleeping sickness, replace their surface proteins to hide from the immune system’s defenses.
“There is not an organism in nature, or a parasite in nature, that has such an enormous repertoire to change its surface,” he said.
Dr. Borst is also credited with the discovery of how some chemotherapy drugs are kept out of the brain and body by transport proteins, forcing physicians to use intravenous methods.
His research has been so wide-ranging “it’s chaotic,” he said.
In recent years Dr. Borst has called out the harms of public health disinformation. He cited President Donald J. Trump’s embrace of ineffective drugs and disinfectants that he wrongly claimed could stop the coronavirus.
“That is a major task for science — is not only to find new things, but also to convince the general public that the kind of facts that we find are not alternative facts but are real facts,” he said.
“Our society, which is getting more and more complex and more science-driven, is absolutely dependent on the scientific method to keep aware of where the truth is, and what is conspiracy theory, quackery or alternative medicine or pseudoscience.”